FNS regularly conducts research and data analysis to inform program or policy decisions and understand nutrition program outcomes. In addition, FNS seeks to make data accessible to state and local agencies, service providers, and the public by developing data visualization and analytics tools that can be used to support nutrition program delivery or report on outcomes.
The below data visualization and analytics products bring together FNS, USDA, and other federal datasets to answer questions related to food security, nutrition assistance programs, and the systems that support them. Dashboards include “about” or “information” pages to answer questions about navigation, interactive functionality, data sources, and the data transformations that have been applied.
National and State-Level Estimates of WIC Eligibility and Program Reach in 2023
This report, the latest in an annual series, presents 2023 national and state-level estimates of the number of people eligible to receive benefits provided through the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and the percentage of the eligible population and the general U.S. population participating in the program.
The number of people eligible for WIC in 2023 remained about the same as the year prior.
- The estimated average monthly WIC-eligible population totaled 11.83 million in calendar year (CY) 2023 (Table 1), essentially unchanged from the estimate of 11.79 million in 2022.
- In 2023, about half of infants and children and more than one third of pregnant women in the U.S. were eligible to participate in WIC.
| Participant Category | Number Eligible | Percent of Total Eligible (%) | Total Population | Eligibility Rate (%) |
|---|---|---|---|---|
| Infants | 1,796,821 | 15.2 | 3,600,389 | 49.9 |
| Children | 7,626,138 | 64.5 | 14,713,756 | 51.8 |
| 1-year-old children | 1,883,018 | 15.9 | 3,672,400 | 51.3 |
| 2-year-old children | 1,914,319 | 16.2 | 3,668,357 | 52.2 |
| 3-year-old children | 1,937,138 | 16.4 | 3,619,422 | 53.5 |
| 4-year-old children | 1,891,664 | 16.0 | 3,753,577 | 50.4 |
| Women | 2,406,255 | 20.3 | 6,615,976 | 36.4 |
| Pregnant women | 1,091,474 | 9.2 | 2,791,608 | 39.1 |
| Postpartum women | 1,314,781 | 11.1 | 3,824,368 | 34.4 |
| Breastfeeding women | 848,829 | 7.2 | 1,995,462 | 42.5 |
| Non-breastfeeding women | 465,952 | 3.9 | 1,828,907 | 25.5 |
| Total | 11,829,215 | 100.0 | 24,930,121 | 47.4 |
In 2023, WIC reached 56% of those who are eligible – the highest coverage rate since 2016.
- The percentage of the eligible population participating in WIC is known as the coverage rate.
- In an average month in 2023, WIC served an estimated 56.1% of those eligible for WIC (Table 2), up from the estimate for 2022 (53.5%), and the highest coverage rate since 2016.
- The increase in the coverage rate resulted from the negligible change in the number of individuals eligible for WIC combined with a significant increase in participation. WIC participation increased by around 320,000 in CY 2023, an increase of about 5% over the year prior.
| Characteristic | Number Eligible | Number Participating | Coverage Rate (%) |
|---|---|---|---|
| Total | 11,829,215 | 6,631,309 | 56.1 |
| Participant Category | |||
| Infants | 1,796,821 | 1,479,155 | 82.3 |
| Children | 7,626,138 | 3,656,078 | 47.9 |
| 1-year-old children | 1,883,018 | 1,268,459 | 67.4 |
| 2-year-old children | 1,914,319 | 1,004,346 | 52.5 |
| 3-year-old children | 1,937,138 | 874,822 | 45.2 |
| 4-year-old children | 1,891,664 | 508,450 | 26.9 |
| Pregnant women | 1,091,474 | 538,332 | 49.3 |
| Postpartum women | 1,314,781 | 957,744 | 72.8 |
| Breastfeeding women | 848,829 | 600,628 | 70.8 |
| Non-breastfeeding women | 465,952 | 357,117 | 76.6 |
| Race and Hispanic Ethnicitya | |||
| Hispanic/Latino | 4,341,509 | 2,865,239 | 66.0 |
| Black-only, not Hispanic | 2,477,494 | 1,308,652 | 52.8 |
| White-only, not Hispanic | 3,742,399 | 1,840,027 | 49.2 |
| Two or more races or other race, not Hispanic | 1,238,566 | 602,318 | 48.6 |
| American Indian/Alaska Native, not Hispanic | 243,653 | 112,539 | 46.2 |
| Asian, not Hispanic | 619,026 | 285,804 | 46.2 |
| Native Hawaiian/Pacific Islander, not Hispanic | 74,125 | 46,583 | 62.8 |
a See report “Table 3.1 WIC coverage rate by participant characteristics” for detailed definitions of race and ethnicity categories used in the report.
Coverage rates varied by participant category and characteristics – continuing to be highest for infants and decreasing as children age.
- In 2023, coverage rates were highest for WIC-eligible infants (82.3%) and postpartum non-breastfeeding women (76.6%), while the coverage rates for WIC-eligible children (47.9%) and pregnant women (49.3%) continued to be lower than other participant categories.
- The relative differences in coverage rates by participant category remained mostly consistent from CY 2005 to CY 2023. Across all years, coverage rates were highest for infants, followed by coverage rates for postpartum women. Coverage rates for children were consistently the lowest (except for 2022, when the coverage rate for pregnant women was the lowest).
- In recent years, coverage rates for pregnant women have declined more rapidly than for other participant categories, declining from 53.0% in 2018 to 49.3% in 2023, despite a small increase in coverage rates for pregnant women between 2021 and 2023.
- The estimated coverage rate for WIC-eligible individuals in metropolitan areas in the average month of 2023 was 61.1%, while the coverage rate for WIC-eligible individuals in nonmetropolitan areas was 24.0%. Of the 11.83 million individuals eligible for WIC, an estimated 10.21 million lived in metropolitan areas in 2023.
A large share of Medicaid and SNAP participants do not participate in WIC despite being eligible.
- Consistent with previous findings, large percentages of Medicaid and Supplemental Nutrition Assistance Program (SNAP) recipients who were eligible for WIC did not participate in WIC in 2023.
- Among WIC-eligible Medicaid participants, 32.0% participated in WIC.
- Among WIC-eligible SNAP participants, 57.6% participated in WIC.
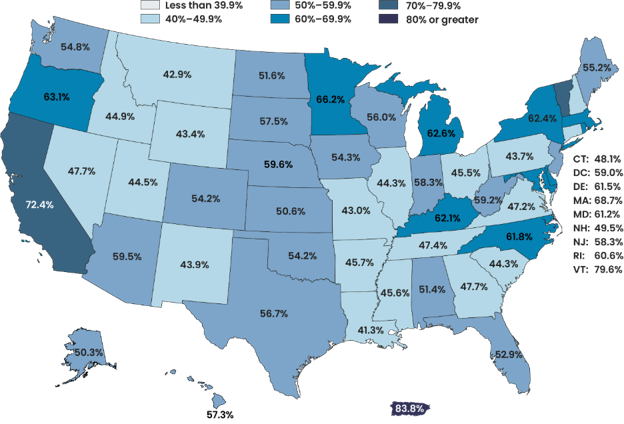
Coverage rates vary substantially by state.
- Coverage rates vary substantially by state, as shown in the map below (Figure 1), ranging from around 40% in some states to more than 70% in others.
- Coverage estimates are less precise for states with small populations compared to other states (see Figure 3.5 in the full report); therefore, differences between states and across years may be less pronounced than they appear in the map.
Why FNS Did This Study
WIC provides healthy foods, breastfeeding support, nutrition education, and referrals to other services to eligible pregnant, breastfeeding and non-breastfeeding (up to six months after the end of pregnancy) postpartum women, infants, and children up to age 5.
WIC funding is provided by Congress through the annual appropriations process. Since approximately 1997, Congress has funded WIC at a level sufficient for the program to serve all eligible applicants. WIC funding needs are estimated annually using the number of individuals eligible for WIC and the percentage of the eligible population likely to participate. We allocate funds to participating state agencies based on a formula that considers the previous year’s funding and the estimated eligible population in each WIC state agency, along with other factors. Accurately estimating the number of individuals eligible for WIC and the number likely to participate enables us to better predict future funding needs, measure WIC performance, and identify potentially unmet nutrition assistance needs.
This report presents estimates of the numbers of women, infants, and children eligible for WIC during an average month in 2023 and historical estimates for 2016–2022. This is the most recent report in a series that provides eligibility estimates at the national, regional, and state levels. Estimates are also provided by participant category—infants, children, pregnant women, and postpartum women—and by race and ethnicity, urbanicity, and reported household income.
How FNS Did This Study
We calculated the estimates for this study on a methodology developed in 2003 by the Committee on National Statistics of the National Research Council.1 The 2023 estimates continue to incorporate methodological improvements first described in the 2021 report.2 These methodologies use data from various sources, including the Community Population Survey Annual Social and Economic Supplement (CPS-ASEC), American Community Survey (ACS), and National Vital Statistics. The estimates presented in this report use the same methodology as and are consistent with the 2022 WIC eligibility estimates published in 2024.3
1 Ver Ploeg, M., & Betson, D. M. (Eds.). (2003). Estimating eligibility and participation for the WIC program: Final report. The National Academies Press.
2 Kessler C., Bryant A., Munkacsy, K., and Gray K. (2023). National- and State-Level Estimates of WIC Eligibility and WIC Program Reach in 2021. Prepared by Insight Policy Research, Contract No 12319819A0005. Alexandria, VA: U.S. Department of Agriculture, Food and Nutrition Service, Office of Policy Support, Project Officer: Grant Lovellette. Available online at: https://www.fns.usda.gov/research/wic/eer/2021.
3 Kessler C., Bryant A., Munkacsy, K., and Gray K. (2024). National- and State-Level Estimates of the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) Eligibility and WIC Program Reach in 2022. Prepared by Insight Policy Research, Contract No 12319819A0005. Alexandria, VA: U.S. Department of Agriculture, Food and Nutrition Service, Office of Policy Support, Project Officer: Grant Lovellette. Available online at: https://www.fns.usda.gov/research/wic/eer/2022.
Suggested Citation
Kessler C., Bryant A., Munkacsy K., Maxson S., Ressler D., Saluja R., and Farson Gray K. (2025). National- and State-Level Estimates of the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) Eligibility and WIC Program Reach in 2023. Prepared by Westat, Contract No. GS-00F-009DA/140D0424A0040, Order No. 140D0424F1045. Alexandria, VA: U.S. Department of Agriculture, Food and Nutrition Service, Project Officer: Grant Lovellette. Available online at: www.fns.usda.gov/research/wic/eer/2023.
This report, the latest in an annual series, presents 2023 national and state-level estimates of the number of people eligible to receive benefits provided through the Special Supplemental Nutrition Program for Women, Infants, and Children and the percentage of the eligible population and the general U.S. population participating in the program.
Comment Request - FNS Generic Clearance for the FNS Fast Track Clearance for the Collection of Routine Customer Feedback
Summary
Under the Government Service Delivery Improvement Act (GSDIA), along with the OMB Circular A-11, Section 280 Implementation, agencies are obligated to continually improve the services they provide the public and to collect qualitative and quantitative data from the public to do so. The Food and Nutrition Service (FNS) seeks to obtain OMB approval for the extension of a generic clearance to collect qualitative feedback on our delivery of services. By qualitative feedback we mean information that provides useful insights on perceptions and opinions but are not statistical surveys that yield quantitative results that can be generalized to the population of study.
Need and Use of the Information
This feedback will continue to: (1) provide insights into customer or stakeholder perceptions, experiences and expectations, (2) provide an early warning of issues with service and, (3) focus attention on areas where communication, training or changes in operations might improve delivery of products or services. This collection allows for ongoing, collaborative, and actionable communications between the Agency and its customers and stakeholders. It also allows feedback to contribute directly to the improvement of program management.
The solicitation of feedback targets areas such as: timeliness, appropriateness, accuracy of information, courtesy, efficiency of service delivery, and resolution of issues with service delivery. Responses are assessed to plan and inform efforts to improve or maintain the quality of service offered to the public. If this information is not collected, vital feedback from customers and stakeholders on the Agency's services will not be available.
The Food and Nutrition Service seeks to obtain OMB approval for the extension of a generic clearance to collect qualitative feedback on our delivery of services.
Semiannual Regulatory Agenda, Spring 2025
Summary
This agenda provides summary descriptions of significant and not significant regulations being developed in agencies of the U.S. Department of Agriculture (USDA) in conformance with Executive Orders (E.O.) 12866, “Regulatory Planning and Review,” 13563, “Improving Regulation and Regulatory Review,” 14192, “Unleashing Prosperity Through Deregulation,” and 14219, “Ensuring Lawful Governance and Implementing the President's “Department of Government Efficiency” Deregulatory Initiative.” The agenda also describes regulations affecting small entities as required by section 602 of the Regulatory Flexibility Act, Public Law 96-354. This agenda also identifies regulatory actions that are being reviewed in compliance with section 610(c) of the Regulatory Flexibility Act. We invite public comments on those actions as well as any regulation consistent with Executive Order 13563.
USDA has attempted to list all regulations and regulatory reviews pending at the time of publication except for minor and routine or repetitive actions, but some may have been inadvertently missed. There is no legal significance to the omission of an item from this listing. Also, the dates shown for the steps of each action are estimated and are not commitments to act on or by the date shown.
USDA's complete regulatory agenda is available online at reginfo.gov. Because publication in the Federal Register is mandated for the regulatory flexibility agendas required by the Regulatory Flexibility Act (5 USC 602), USDA's printed agenda entries include only:
- Rules that are likely to have a significant economic impact on a substantial number of small entities; and
- Rules identified for periodic review under section 610 of the Regulatory Flexibility Act.
Food and Nutrition Service - Proposed Rule Stage
| Sequence No. | Title | Regulation Identifier No. |
|---|---|---|
| 13 | Strengthening Integrity and Reducing Retailer Fraud in the Supplemental Nutrition Assistance Program (SNAP) | 0584-AE71 |
| 14 | Updated Staple Food Stocking Standards for Retailers in the Supplemental Nutrition Assistance Program | 0584-AF12 |
Food and Nutrition Service - Final Rule Stage
| Sequence No. | Title | Regulation Identifier No. |
|---|---|---|
| 15 | Special Supplemental Nutrition Program for Women, Infants and Children (WIC): WIC Online Ordering and Transactions and Food Delivery Revisions to Meet the Needs of a Modern, Data-Driven Program | 0584-AE85 |
13. Strengthening Integrity and Reducing Retailer Fraud in the Supplemental Nutrition Assistance Program (SNAP)
Legal Authority: PL 113-79; PL 115-334
Abstract: This proposed rule would implement statutory provisions of the Food, Conservation, and Energy Act of 2008 (the 2008 Farm Bill), the Agriculture Improvement Act of 2018 (the 2018 Farm Bill), and other language intended to deter retailer fraud, abuse, and non-compliance in the Supplemental Nutrition Assistance Program (SNAP). Stakeholders are SNAP retailers and communities in which SNAP retailers provide SNAP participants access to food, other programs that require SNAP authorization or where reciprocal actions impact participation, and SNAP participants.
Timetable: Action - NPRM; Date - 02/00/26
Regulatory Flexibility Analysis Required: Yes
RIN: 0584-AE71
14. Updated Staple Food Stocking Standards for Retailers in the Supplemental Nutrition Assistance Program
Legal Authority: PL 113-79; 7 USC 2011 to 2036
Abstract: The Agricultural Act of 2014 amended the Food and Nutrition Act of 2008 to increase the requirement that certain Supplemental Nutrition Assistance Program (SNAP) authorized retail food stores have available on a continuous basis at least three varieties of items in each of food staple food categories, to a mandatory minimum of seven varieties. This proposed rule would provide some retailers participating in SNAP as authorized food stores with more flexibility in meeting the enhanced SNAP eligibility requirements while also simplifying the criteria.
Timetable: Action - NPRM; Date- 02/00/26
Regulatory Flexibility Analysis Required: Yes
RIN: 0584-AF12
15. Special Supplemental Nutrition Program for Women, Infants, and Children (WIC): WIC Online Ordering and Transactions and Food Delivery Revisions to Meet the Needs of a Modern, Data-Driven Program
Legal Authority: PL 111-296
Abstract: This “final rule with comment” addresses key regulatory barriers to online ordering in the WIC Program by making changes to the provisions that prevent online transactions and types of online capable stores from participating in the program. This rule will also allow FNS to modernize WIC vendor regulations that do not reflect current technology and facilitate the Program's transition to Electronic Benefit Transfer (EBT). The final rule is responsive to prior proposed rule public comments from WIC state, public and private industry stakeholders to ensure that the final rule reflects their substantive feedback as online shopping and FNS' modernization efforts are made permanent.
Timetable:
| Action | Date | FR Cite |
|---|---|---|
| NPRM NPRM Comment Period End Final Action | 02/23/23 05/24/23 02/00/26 | 88 FR 11516 |
Regulatory Flexibility Analysis Required: Yes
RIN: 0584-AE85
This agenda provides summary descriptions of significant and not significant regulations being developed in agencies of the USDA in conformance with Executive Orders 12866, “Regulatory Planning and Review,” 13563, “Improving Regulation and Regulatory Review,” 14192, “Unleashing Prosperity Through Deregulation,” and 14219, “Ensuring Lawful Governance and Implementing the President's “Department of Government Efficiency” Deregulatory Initiative.”
Request for Information: Ultra-Processed Foods - Extension of Comment Period
Summary
FDA and USDA (we) are extending the comment period for the notice that appeared in the Federal Register of July 25, 2025. In the notice, we requested data and information to help develop a uniform definition of ultra-processed foods (UPF or UPFs). In response to requests for an extension, we are extending the comment period until Oct. 23, 2025, to allow interested persons additional time to submit comments.
Request for Comments
We are extending the comment period announced in the notice published July 25, 2025 (90 FR 35305). Electronic or written comments must be submitted by Oct. 23, 2025.
You may submit comments as follows. Please note that late, untimely filed comments will not be considered. The Regulations.gov electronic filing system will accept comments until 11:59 p.m. ET at the end of Oct. 23, 2025. Comments received by mail/hand delivery/courier (for written/paper submissions) will be considered timely if they are received on or before that date.
Electronic Submissions
Submit electronic comments in the following way:
- Federal eRulemaking Portal: Regulations.gov. Follow the instructions for submitting comments. Comments submitted electronically, including attachments, to Regulations.gov will be posted to the docket unchanged. Because your comment will be made public, you are solely responsible for ensuring that your comment does not include any confidential information that you or a third party may not wish to be posted, such as medical information, your or anyone else's Social Security number, or confidential business information, such as a manufacturing process. Please note that if you include your name, contact information, or other information that identifies you in the body of your comments, that information will be posted on Regulations.gov.
- If you want to submit a comment with confidential information that you do not wish to be made available to the public, submit the comment as a written/paper submission and in the manner detailed (see “Written/Paper Submissions” and “Instructions”).
Written/Paper Submissions
Submit written/paper submissions as follows:
- Mail/Hand delivery/Courier (for written/paper submissions): Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852.
- For written/paper comments submitted to the Dockets Management Staff, FDA will post your comment, as well as any attachments, except for information submitted, marked and identified, as confidential, if submitted as detailed in “Instructions.”
Instructions: All submissions received must include the Docket No. FDA-2025-N-1793 for “Ultra-Processed Foods; Request for Information.” Received comments, those filed in a timely manner, will be placed in the docket and, except for those submitted as “Confidential Submissions,” publicly viewable at Regulations.gov or at the Dockets Management Staff between 9 a.m. and 4 p.m., Monday through Friday, 240-402-7500.
- Confidential Submissions—To submit a comment with confidential information that you do not wish to be made publicly available, submit your comments only as a written/paper submission. You should submit two copies total. One copy will include the information you claim to be confidential with a heading or cover note that states “THIS DOCUMENT CONTAINS CONFIDENTIAL INFORMATION.” We will review this copy, including the claimed confidential information, in our consideration of comments. The second copy, which will have the claimed confidential information redacted/blacked out, will be available for public viewing and posted on Regulations.gov. Submit both copies to the Dockets Management Staff. If you do not wish your name and contact information to be made publicly available, you can provide this information on the cover sheet and not in the body of your comments and you must identify this information as “confidential.” Any information marked as “confidential” will not be disclosed except in accordance with 21 CFR 10.20 and other applicable disclosure law. For more information about FDA's posting of comments to public dockets, see 80 FR 56469, Sept. 18, 2015.
Docket: For access to the docket to read background documents or the electronic and written/paper comments received, go to Regulations.gov and insert the docket number, found in brackets in the heading of this document, into the “Search” box and follow the prompts or go to the Dockets Management Staff, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852, 240-402-7500.
Supplementary Information
In the Federal Register of July 25, 2025, FDA and USDA published a notice requesting data and information to help develop a uniform definition of ultra-processed foods for human food products in the U.S. food supply (90 FR 35305). A uniform UPF definition, developed as part of a joint effort by federal agencies, would allow for consistency in research and policy to pave the way for addressing health concerns associated with the consumption of UPFs. The notice requested comments by Sept. 23, 2025.
We have received requests to extend the comment period for the notice. Pointing to the complexity of the questions, the importance of the issue, and other factors, the requests assert that additional time would allow stakeholders to provide FDA and USDA detailed responses. We have considered the requests and are extending the comment period for the notice by 30 days, until Oct. 23, 2025. We believe that the extension will allow adequate time for interested persons to submit comments.
FDA and USDA (we) are extending the comment period for the notice that appeared in the Federal Register of July 25, 2025. In the notice, we requested data and information to help develop a uniform definition of ultra-processed foods. In response to requests for an extension, we are extending the comment period until Oct. 23, 2025, to allow interested persons additional time to submit comments.
FD-162: Public Posting of TEFAP Information
| DATE: | September 9, 2025 | |
|---|---|---|
| POLICY NO: | FD-162: The Emergency Food Assistance Program (TEFAP) | |
| SUBJECT: | Public Posting of TEFAP Information | |
| TO: | Regional Directors Supplemental Nutrition Programs | State Directors All TEFAP State Agencies |
Under the leadership of Secretary Brooke Rollins, the U.S. Department of Agriculture’s (USDA) Food and Nutrition Service (FNS) is committed to strengthening strategies to encourage healthy choices, healthy outcomes, and healthy families, along with clarifying program requirements for our state agency partners. In support of these goals, this memorandum provides guidance to TEFAP state agencies on requirements for public posting of TEFAP information at 7 CFR 251.4(l). Each state agency must post a list of eligible recipient agencies (ERAs) that have an agreement with the state agency on a publicly available webpage. In addition, state agencies must post the state’s uniform statewide eligibility criteria on a publicly available webpage. The public posting of ERAs and uniform statewide eligibility criteria must be implemented by Oct. 31, 2025. State agencies are encouraged to implement these provisions before that deadline.
ERAs that have an Agreement with the State Agency
TEFAP regulations at 7 CFR 251.4(l) require each state agency to post a list of ERAs that have an agreement with the state agency on a publicly available webpage. At a minimum, this list must include the name, address, and contact telephone number of all ERAs that have an agreement with the state agency. State agencies must update this list annually but are encouraged to update it more frequently as needed.
FNS recognizes that state agencies may identify a compelling public safety reason to forgo posting an ERA address publicly; for example, for a domestic abuse shelter. In such circumstances and without divulging sensitive or confidential information, the state agency should submit general information to their FNS regional office regarding why the location should be exempted from the publicly available list posted online. FNS will work with state agencies on a case-by-case basis for ERAs in this situation.
State Agency Option to Post Additional ERA Information
State agencies may choose to exceed the above minimum posting requirements to support public awareness. While state agencies are not required to post information about ERAs that have agreements with other ERAs, states have the option to publish this information online. State agencies may also choose to include additional information about ERAs on the webpage, such as operating hours, the areas served by the ERA, links to ERA websites, and distribution site addresses. In addition, state agencies may develop tools to aid eligible individuals in accessing the program, for example by establishing a searchable tool to identify aid based on zip code.
Posting TEFAP Statewide Eligibility Information
TEFAP regulations at 7 CFR 251.5(b) require each state agency to establish uniform statewide criteria for determining the eligibility of households to receive USDA Foods for home consumption, including requirements for demonstrating income and residency. Per 7 CFR 251.4(l), state agencies must post this uniform statewide eligibility criteria to a publicly available webpage. This information must be updated on an annual basis or whenever changes to eligibility criteria are made. Additional guidance on establishing criteria and methods for determining the eligibility of households to receive TEFAP can be found in Participant Eligibility in TEFAP (revised).
State agencies should contact their respective FNS regional office with any questions about this memorandum.
Sara Olson
Director
Policy Division
Supplemental Nutrition and Safety Programs
This TEFAP program guidance memorandum provides TEFAP state agencies information on requirements for public posting of TEFAP information.
MAHA Commission Unveils Sweeping Strategy to Make Our Children Healthy Again
Washington, Sept. 9, 2025 – The Make America Healthy Again Commission today released the Make Our Children Healthy Again Strategy, a sweeping plan with more than 120 initiatives to reverse the failed policies that fueled America’s childhood chronic disease epidemic. The strategy outlines targeted executive actions to advance gold-standard science, realign incentives, increase public awareness, and strengthen private-sector collaboration.
Chaired by U.S. Health and Human Services Secretary Robert F. Kennedy Jr., the Commission is tasked with investigating and addressing the root causes of America’s escalating health crisis, with a focus on childhood chronic diseases.
“The Trump Administration is mobilizing every part of government to confront the childhood chronic disease epidemic,” Secretary Kennedy said. “This strategy represents the most sweeping reform agenda in modern history—realigning our food and health systems, driving education, and unleashing science to protect America’s children and families. We are ending the corporate capture of public health, restoring transparency, and putting gold-standard science—not special interests—at the center of every decision.”
“Today’s MAHA Commission report is another historic milestone for our country and a testament to President Trump’s leadership and commitment to Make America Healthy Again,” said U.S. Secretary of Agriculture Brooke L. Rollins. “America’s farmers and ranchers are at the heart of the solution — alongside doctors, parents, and communities - to fight chronic disease and protect future generations. Under this Administration, we are not just talking about healthy outcomes; we are delivering them by securing voluntary commitments to remove artificial food dye from major brands, providing technical assistance to states interested in restricting junk food and soda from SNAP, and providing growers with new tools to maintain and improve soil health, including the introduction of a regenerative farming practice pilot program. Together with our partners at HHS and EPA, we are charting a new course, strengthening the health of our families, and ensuring the United States leads the world with the safest, strongest, and most abundant food supply.”
Key Focus Areas of the Strategy
Restoring Science & Research: Expanding NIH and agency research into chronic disease prevention, nutrition and metabolic health, food quality, environmental exposures, autism, gut microbiome, precision agriculture, rural and tribal health, vaccine injury, and mental health.
Historic Executive Actions: Reforming dietary guidelines; defining ultra-processed foods; improving food labeling; closing the GRAS loophole; raising infant formula standards; removing harmful chemicals from the food supply; increasing oversight and enforcement of direct-to-consumer prescription drug advertising laws; improving food served in schools, hospitals, and to veterans; and reforming Medicaid quality metrics to measure health outcomes.
Process Reform & Deregulation: Streamlining organic certification; easing barriers to farm-to-school programs and direct-to-consumer sales; restoring whole milk in schools; supporting mobile grocery and processing units; modernizing FDA drug and device approval; and accelerating EPA approvals for innovative agricultural products.
Public Awareness & Education: Launching school-based nutrition and fitness campaigns, Surgeon General initiatives on screen time, prioritizing pediatric mental health, and expanding access to reliable nutrition and health information for parents.
Private Sector Collaboration: Promoting awareness of healthier meals at restaurants, soil health and land stewardship, and community-led initiatives, and scaling innovative solutions to address root causes of chronic disease.
With this strategy, the MAHA Commission leads the most ambitious national effort ever to confront childhood chronic disease and Make America Healthy Again.
“Protecting human health and the environment while powering America's comeback isn’t just about serving Americans today; it’s about ensuring future generations inherit clean air, land, water, and the foundation for healthy lives,” EPA Administrator Zeldin said. “The Make America Healthy Again strategy outlines the keys to success from pro-growth policies that advance research and drive innovation to private sector collaboration and increased public awareness. I look forward to continuing to work collaboratively across the federal family to ensure our kids and our environment are protected.”
“For too long health care has used a reactive approach to chronic diseases,” FDA Commissioner Dr. Marty Makary said. “I am pleased to support the findings of the MAHA commission and to promote a more proactive approach, tackling root causes undermining the health and happiness of American children.”
“The MAHA Report provides a blueprint for the entire government to focus on solving the chronic disease crisis facing American children,” NIH Director Dr. Jay Bhattacharya said. “We must make America healthy again so our children live longer and healthier lives than we will.”
Today’s MAHA Commission press event included: HHS Secretary Robert F. Kennedy, Jr., DPC Director Vince Haley, USDA Secretary Brooke Rollins, EPA Administrator Lee Zeldin, NIH Director Dr. Jay Bhattacharya, FDA Commissioner Dr. Marty Makary, HHS Deputy Secretary Jim O’Neill, NEC Director Kevin Hassett, CEA Vice Chair Pierre Yared, and OSTP Director Michael Kratsios.
###
Written Information on and Referrals to Public Assistance Programs for CSFP Participants
| DATE: | August 25, 2025 | |
|---|---|---|
| POLICY NO: | FD-161: Commodity Supplemental Food Program (CSFP) | |
| SUBJECT: | Written Information on and Referrals to Public Assistance Programs for CSFP Participants | |
| TO: | Regional Directors Supplemental Nutrition Programs | Directors CSFP State Agencies and Indian Tribal Organizations (ITOs) |
Under the leadership of Secretary Brooke Rollins, USDA’s Food and Nutrition Service (FNS) is prioritizing the clarification of statutory, regulatory, and administrative requirements, as well as strengthening strategies to encourage healthy choices, healthy outcomes, and healthy families. In support of these goals, FNS is issuing this memorandum to provide CSFP state agencies, including ITOs, with guidance on implementing 7 CFR § 247.14(a), which requires local agencies, as appropriate, to make referrals and provide CSFP applicants with written information on specific public assistance programs. The specific public assistance programs are:
- supplemental security income benefits (SSI);
- medical assistance under Title XIX of the Social Security Act, including medical assistance provided to qualified Medicare beneficiaries;
- the Supplemental Nutrition Assistance Program (SNAP); and
- beginning on Oct. 31, 2025, the Senior Farmers’ Market Nutrition Program (SFMNP).
Methods of Information Sharing
CSFP local agencies may provide written materials in hard copy or via electronic means such as a link to a webpage, an email, or text messages to applicants.
State and Local Agency Coordination
FNS recommends that, as applicable, CSFP state agencies coordinate with the appropriate state agency for each public assistance program to ensure written information for local agency dissemination is accurate and up to date. Coordination may also occur between CSFP local agencies and entities administering the identified public assistance programs at the local level; for example, between a CSFP local agency and the local Social Security Office. For further information and applicable state or local agency contacts, see below:
- SSI
- Medicaid and Medicare
- SNAP
- SFMNP
Considerations for the Senior Farmers' Market Nutrition Program
SFMNP is a federal nutrition assistance program that provides low-income seniors with seasonal benefits that can be exchanged for eligible foods directly from farmers, farmers’ markets, roadside stands, and community-supported agriculture programs (CSAs). CSFP and SFMNP work in tandem to serve the low-income senior population and help meet their nutritional needs. Connecting CSFP participants with SFMNP creates new opportunities for American farmers to connect with federal nutrition assistance programs.
SFMNP is administered by state agencies, which are responsible for determining months of operation, as the program may not be available year-round. SFMNP is not available nationwide and, in states where it does operate, may not be available in all areas of a state. CSFP state and local agencies may use discretion on when it is appropriate to distribute materials and make referrals to SFMNP based on program availability.
CSFP state agencies should coordinate with SFMNP state agencies to access up-to-date information on SFMNP and determine whether the program is accepting new participants within the state. If SFMNP is not available in the applicable area(s) or the state’s SFMNP is not accepting participants, then CSFP local agencies do not need to provide written information and/or referrals to that program.
State agencies should contact their respective FNS regional office with any questions about this memorandum.
Sara Olson
Director
Policy Division
Supplemental Nutrition and Safety Programs
We are issuing this memorandum to provide CSFP state agencies, including ITOs, with guidance on implementing 7 CFR § 247.14(a), which requires local agencies, as appropriate, to make referrals and provide CSFP applicants with written information on specific public assistance programs.
Information Collection: WIC and Senior Farmers' Market Nutrition Programs - Reporting and Recordkeeping Burden
Summary
In accordance with the Paperwork Reduction Act of 1995, this notice invites the general public and other public agencies to comment on this proposed information collection. This collection is: (1) a revision of the currently approved collections for the reporting and recordkeeping burdens associated with the Senior Farmers' Market Nutrition Program (SFMNP) regulations and with the Women, Infants, and Children (WIC) Farmers' Market Nutrition Program (FMNP) regulations; and (2) a consolidation of the SFMNP and WIC FMNP reporting and recordkeeping burdens into a single information collection to more accurately reflect consolidated program operations.
Request for Comments
Written comments must be received on or before Oct. 7, 2025.
The Food and Nutrition Service, USDA, invites interested persons to submit written comment.
- Preferred Method: Federal eRulemaking Portal. Go to http://www.regulations.gov and follow the online instructions for submitting comments electronically.
- Mail: Allison Post, Food and Nutrition Service, U.S. Department of Agriculture, 1320 Braddock Place, Room 328, Alexandria, VA 22302.
- Email: Send email to allison.post@usda.gov.
All responses to this notice will be summarized and included in the request for Office of Management and Budget (OMB) approval. All comments will be a matter of public record.
Abstract
The purpose of the Senior Farmers' Market Nutrition Program (SFMNP) is to provide resources in the form of fresh, nutritious, unprepared, locally grown fruits, vegetables, herbs, and honey from farmers' markets, roadside stands, and community supported agriculture (CSA) programs to low income seniors; to increase the domestic consumption of agricultural commodities by expanding or aiding in the expansion of domestic farmers' markets, roadside stands, and CSA programs; and to develop or aid in the development of new and additional farmers' markets, roadside stands, and CSA programs. SFMNP is administered by state agencies in 45 states, eight Indian Tribal Organizations, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands.
The WIC Farmers' Market Nutrition Program (FMNP) is associated with the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). WIC provides supplemental foods, health care referrals, and nutrition education at no cost to low-income pregnant, breastfeeding, and non-breastfeeding postpartum participants, infants, and children up to 5 years of age at nutritional risk. The purpose of WIC FMNP is to provide fresh, nutritious, unprepared, locally grown fruits and vegetables through farmers' markets and roadside stands to WIC participants, and to expand awareness and use of, and sales at, farmers' markets and roadside stands. WIC FMNP is administered by state agencies in 40 states, seven Indian Tribal Organizations, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands.
WIC FMNP and SFMNP have near-identical program requirements and are often administered by the same state agency as a “consolidated” program. State agencies administering consolidated programs may accept a single application from a farmer, farmers' market, or roadside stand, for participation in both programs. Additionally, consolidated state agencies may combine farmer, farmers' market, and roadside stand monitoring and evaluation efforts, and may use the same coupon or electronic benefit management system for both programs allowing for combined maintenance and recordkeeping efforts. Consolidated WIC FMNP and SFMNP programs are administered by 23 states, six Indian Tribal Organizations, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands.
SFMNP statute (7 USC 3007) and regulations (7 CFR part 249), and WIC FMNP statute (42 USC 1786(m)(8)) and regulations (7 CFR part 248), require that certain program-related information be collected and that full and complete records concerning program operations are maintained. The information reporting and recordkeeping requirements are necessary to ensure appropriate and efficient management of both programs. The burden activities that are covered by this Information Collection Request (ICR) include requirements that involve the authorization and monitoring of local agencies; the certification of participants; the nutrition education that is provided to participants; farmer, farmers' market, roadside stand, and CSA program (SFMNP only) authorization, training, monitoring, and management; and financial and participation data.
State Plans are the principal source of information about how each state agency operates WIC FMNP and SFMNP. State agencies administering both programs may submit a single consolidated State Plan describing both WIC FMNP and SFMNP operations to the U.S. Department of Agriculture's (USDA) Food and Nutrition Service (FNS) (7 CFR 249.4(a)). State Plans are currently submitted to FNS electronically as Word or PDF documents using a web-based application called PartnerWeb. FNS's Waivers and State Plans (WiSP) application will allow state agencies to directly enter and submit State Plan information to FNS. Once live, the reporting and recordkeeping burdens associated with state agencies inputting and storing information in WiSP will be captured in the WiSP ICR (OMB Control Number not yet determined; see 89 FR 106420). FNS expects that, beyond the burdens covered in the WiSP ICR, state agencies will spend additional time collecting and recording information from local agencies and authorized outlets in preparation for their State Plan submissions, and therefore this ICR maintain some State Plan-related reporting and recordkeeping burden.
Information from participants and local agencies is collected through state agency-developed forms or Management Information Systems. The information collected is used by FNS to manage, plan, evaluate, make decisions and report on SFMNP and WIC FMNP operations. Along with State Plans, all state agencies also submit the Federal-State Supplemental Nutrition Programs Agreement (FNS-339), for which the associated reporting and recordkeeping burden is approved under OMB Control Number: 0584-0332, Expiration Date: 7/31/2025.
Additionally, SFMNP financial and participation data are collected using the SFMNP Annual Financial and Program Data Report (FNS 683A), and WIC FMNP financial and participation data are collected using the WIC FMNP Annual Financial and Program Data Report (FNS 683B). These forms and their associated reporting burdens are approved under OMB Control Number: 0584-0594 Food Programs Reporting System (FPRS), Expiration Date: 9/30/2026. The recordkeeping burdens associated with forms FNS 683A and FNS 683B are not approved under OMB Control Number 0584-0594. State agencies must maintain records to support data reported in FPRS, and the recordkeeping burden for such record maintenance is captured in this ICR, OMB Control Number: 0584-0447 (and previously in OMB Control Number 0584-0541).
With this information collection, FNS is requesting a revision in the burden hours due to program changes and program adjustments. The most significant program changes reported in this revision are due to SFMNP state agencies transitioning from paper coupon systems to electronic benefit systems, and more accurate reporting of consolidated state agencies' burdens. Additional program changes include the creation of the WiSP application for WIC FMNP and SFMNP state agencies to submit State Plans; corrections to align estimates between programs or more accurately capture program requirements; and corrections to account for existing requirements that have been in use without Paperwork Reduction Act approval. The program adjustments account for changes in the number of participants, authorized outlets (farmers, farmers' markets, roadside stands, and—for SFMNP only—CSA programs), and state and local agencies across both programs.
To date, the WIC FMNP reporting and recordkeeping requirements have been approved under OMB Control Number 0584-0447 (expiration date: 8/31/2027), and the SFMNP reporting and recordkeeping requirements have been separately approved under OMB Control Number 0584-0541 (expiration date: 1/31/2026). This revision proposes to consolidate the SFMNP ICR and the WIC FMNP ICR into a single collection under OMB Control Number 0584-0447. Consolidating the two ICRs will allow FNS to more accurately and clearly capture the two programs' information collection burdens, place both programs on the same cycle of ICR renewals and reduce administrative inefficiencies at FNS.
The currently approved burden for the SFMNP collection is 1,137,363 hours, and the currently approved burden for the WIC FMNP collection is 1,175,964 hours (2,313,327 hours combined). FNS estimates the new, combined burden at 1,760,175 burden hours, which is a decrease of 553,152 hours. The currently approved number of responses for the SFMNP collection is 2,401,277 total annual responses, and the currently approved number of responses for the WIC FMNP collection is 4,149,393 total annual responses (6,550,670 annual responses combined). FNS estimates the new, combined number of responses at 6,965,338, which is an increase of 414,668 total annual responses.
This collection is: (1) a revision of the currently approved collections for the reporting and recordkeeping burdens associated with the Senior Farmers' Market Nutrition Program (SFMNP) regulations and with the Women, Infants, and Children (WIC) Farmers' Market Nutrition Program (FMNP) regulations; and (2) a consolidation of the SFMNP and WIC FMNP reporting and recordkeeping burdens into a single information collection to more accurately reflect consolidated program operations.
Request for Information: Ultra-Processed Foods
Extension of Comment Period
In response to requests for an extension, we are extending the comment period until Oct. 23, 2025, to allow interested persons additional time to submit comments.
Summary
FDA and USDA (we) are requesting data and information to help develop a uniform definition of ultra-processed foods (UPF or UPFs) for human food products in the U.S. food supply. A uniform UPF definition, developed as part of a joint effort by federal agencies, would allow for consistency in research and policy to pave the way for addressing health concerns associated with the consumption of UPFs.
Request for Comments
Either electronic or written comments on the notice must be submitted by Sept. 23, 2025.
You may submit comments and information as follows. Please note that late, untimely filed comments will not be considered. The https://www.regulations.gov electronic filing system will accept comments until 11:59 p.m. Eastern Time at the end of Sept. 23, 2025. Comments received by mail/hand delivery/courier (for written/paper submissions) will be considered timely if they are received on or before that date.
Electronic Submissions
Submit electronic comments in the following way:
- Federal eRulemaking Portal: https://www.regulations.gov. Follow the instructions for submitting comments. Comments submitted electronically, including attachments, to https://www.regulations.gov will be posted to the docket unchanged. Because your comment will be made public, you are solely responsible for ensuring that your comment does not include any confidential information that you or a third party may not wish to be posted, such as medical information, your or anyone else's Social Security number, or confidential business information, such as a manufacturing process. Please note that if you include your name, contact information, or other information that identifies you in the body of your comments, that information will be posted on https://www.regulations.gov.
- If you want to submit a comment with confidential information that you do not wish to be made available to the public, submit the comment as a written/paper submission and in the manner detailed (see “Written/Paper Submissions” and “Instructions”).
Written/Paper Submissions
Submit written/paper submissions as follows:
- Mail/Hand delivery/Courier (for written/paper submissions): Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852.
- For written/paper comments submitted to the Dockets Management Staff, FDA will post your comment, as well as any attachments, except for information submitted, marked and identified, as confidential, if submitted as detailed in “Instructions.”
Instructions: All submissions received must include the Docket No. FDA-2025-N-1793 for “Ultra-Processed Foods; Request for Information.” Received comments, those filed in a timely manner, will be placed in the docket and, except for those submitted as “Confidential Submissions,” publicly viewable at https://www.regulations.gov or at the Dockets Management Staff between 9 a.m. and 4 p.m., Monday through Friday, 240-402-7500.
- Confidential Submissions—To submit a comment with confidential information that you do not wish to be made publicly available, submit your comments only as a written/paper submission. You should submit two copies total. One copy will include the information you claim to be confidential with a heading or cover note that states “THIS DOCUMENT CONTAINS CONFIDENTIAL INFORMATION.” We will review this copy, including the claimed confidential information, in our consideration of comments. The second copy, which will have the claimed confidential information redacted/blacked out, will be available for public viewing and posted on https://www.regulations.gov. Submit both copies to the Dockets Management Staff. If you do not wish your name and contact information to be made publicly available, you can provide this information on the cover sheet and not in the body of your comments and you must identify this information as “confidential.” Any information marked as “confidential” will not be disclosed except in accordance with 21 CFR 10.20 and other applicable disclosure law. For more information about FDA's posting of comments to public dockets, see 80 FR 56469, Sept. 18, 2015, or access the information at: https://www.govinfo.gov/content/pkg/FR-2015-09-18/pdf/2015-23389.pdf.
Docket: For access to the docket to read background documents or the electronic and written/paper comments received, go to https://www.regulations.gov and insert the docket number, found in brackets in the heading of this document, into the “Search” box and follow the prompts or go to the Dockets Management Staff, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852, 240-402-7500.
Supplementary Information
I. Background
The United States faces a growing epidemic of preventable diet-related chronic diseases, such as cardiovascular disease, and type 2 diabetes, which are leading causes of death and disability in the U.S. (Ref. 1). Improving nutrition is therefore one of the most important public health interventions for reducing chronic illnesses and premature death, and for helping make Americans healthier.
Over the last decade, concerns have grown significantly about the increased availability and consumption of foods that researchers have termed “ultra-processed.” Researchers have found links between consumption of these foods and a range of negative health outcomes, including cardiovascular disease, obesity, and certain cancers (see, e.g., Refs. 2, 3, 4). Consumption of these foods may also be associated with lower diet quality, increased caloric intake, and the intake of food additives (see, e.g., Refs. 5, 6, 7). Some researchers have estimated that more than half of calories consumed by adults and children in the U.S. are from foods that the researchers classified as ultra-processed (Refs. 8, 9).
In May 2025, the President's Make America Healthy Again (MAHA) Commission released “The MAHA Report: Make Our Children Healthy Again: Assessment” (MAHA Report) (Ref. 7). Among other topics, the MAHA Report highlights the prevalence of certain processed foods in the U.S. food system and notes the health concerns associated with their consumption (Ref. 7; see also Refs. 8, 9). FDA and the National Institutes of Health (NIH) have also announced plans to invest in gold standard science through the new NIH-FDA Nutrition Regulatory Science Program to help better understand how and why consumption of ultra-processed foods can harm people's health (Ref. 10).
There is no single, universally accepted definition of UPFs, and the definition of such foods has varied considerably over time (see, e.g., Ref. 11). Classification systems may use either the terms “ultra-processed” or “highly processed,” and the classification of a food can vary between systems due to differing approaches to the definition (Refs. 12, 13).
The most common classification, developed by Brazilian researchers in 2009, is the “Nova” system (Ref. 14). In its latest iteration, the Nova system classifies foods into four food categories: group 1, unprocessed or minimally processed foods; group 2, processed culinary ingredients; group 3, processed foods; and group 4, ultra-processed foods (Ref. 15). The Nova system identifies ultra-processed foods (group 4) based on multiple factors; these factors include things like the use of certain ingredients and substances (such as emulsifiers, bulking agents, or thickeners), industrial processing technologies, as well as sophisticated packaging, that result in a palatable and appealing product (Refs. 15, 16, 17).
However, concerns have been raised about the full ability of UPF classification systems to accurately capture the characteristics of UPFs that may impact health. For example, on one hand, there is overlap between foods considered to be ultra-processed and foods that are high in added sugars, sodium, and saturated fat, which independently are recommended to be limited by the Dietary Guidelines for Americans, 2020-2025 (Refs. 6, 18). Foods commonly considered to be ultra-processed encompass a broad range of industrially processed foods, such as soft drinks and many packaged snacks.
On the other hand, foods considered to be ultra-processed may also include foods such as whole grain products or yogurt, which are known to have beneficial effects on health and are recommended as part of healthy dietary patterns (see Ref. 18). It is important therefore to consider unintended consequences of an overly-inclusive definition of UPFs that could discourage intake of potentially beneficial foods.
Recently, some U.S. states have sought to establish their own definitions of “ultra-processed foods,” with proposed definitions varying. These proposed state definitions include, among others:
- Proposals to define UPFs as foods that include substances intended to have a certain effect on food (such as stabilizers and thickeners, coloring or flavoring agents) (see,e.g., Pennsylvania, 2025 Bill Text PA H.B. 1132; California, 2025 Bill Text CA A.B. 1264);
- Proposals to define UPFs as foods that have undergone certain processing steps (such as hydrogenation of oils or hydrolysis of proteins) (see,e.g., Massachusetts, 2025 Bill Text MA H.B. 539); and
- Proposals to define UPFs as foods that include one of anywhere between 10 and 15 listed ingredients (see,e.g., Florida, 2025 Bill Text FL S.B. 1826 (seeking to define UPFs as foods that include one of 11 listed ingredients); Louisiana, 2025 Bill Text LA S.B. 117 (seeking to define UPFs as foods that include one of 15 listed ingredients); North Carolina, 2025 Bill Text NC H.B. 874 (seeking to define UPFs as foods that include one of 11 listed ingredients); Arkansas, 2025 Bill Text AR H.B. 1962 (seeking to define UPFs as foods that contain one of 10 listed ingredients); Alabama, 2025 Bill Text AL H.B. 580 (seeking to define UPFs as foods that contain one of 11 listed ingredients); South Carolina, 2025 Bill Text SC S.B. 589 (seeking to define UPFs as foods that contain one of 11 listed ingredients); Kentucky, 2025 Bill Text KY H.B. 439 (seeking to define UPFs as foods that contain one of 11 listed ingredients)).
Additionally, some third-party organizations are starting to develop their own definitions for UPFs.
There is a clear need for a uniform definition of UPFs to allow for consistency in research and policy. With this Request for Information, we seek data and information that would enable us, as part of a joint federal agency effort, to define UPFs.
II. Issues for Consideration and Request for Information
We invite comment on the questions below. Please explain your answers and provide references and data, if possible. To the extent that you rely on an existing definition of UPFs (or a facet of such definition) to inform your responses, please state which specific definition it is.
- What, if any, existing classification systems or policies should we consider in defining UPFs? What are the advantages and challenges in applying these systems (or aspects of them) to classify a food as ultra-processed? What are characteristics that would or would not make a given system (or aspect of the system) particularly suitable for the U.S. food supply? Please provide supporting data and explain your rationale in your response.
- FDA-required ingredient labeling provides important information to consumers about what is in packaged foods. The ingredient declaration on a food label lists each ingredient by its common or usual name (21 CFR 101.4(a)(1)). This ingredient name sometimes provides information on specific forms of the ingredient used, such as “flour” versus “whole grain flour.” Additionally, ingredients are declared in descending order of predominance by weight (21 CFR 101.4(a)), which may help a consumer determine the relative proportion of whole versus processed ingredients. For certain types of ingredients, such as flavorings, colorings, and chemical preservatives, labeling must also provide the function of the ingredient (see 21 CFR 101.22). The following questions focus on the ingredient list on the labeling of packaged foods.
- In considering ingredients that appear toward the beginning of an ingredient list (that is, ingredients that likely form most of a finished food by weight), what types of ingredients (e.g., ingredients that may share a similar composition, function, or purpose) might be used to characterize a food as ultra-processed? Please provide supporting data and explain your rationale in your response.
- Ingredients that appear toward the end of an ingredient list may contribute minimally to the overall composition and weight of a finished food (for example, ingredients may sometimes be listed as containing 2% or less by weight of the finished food (21 CFR 101.4(a)(2))). What types of these less prominent ingredients (e.g., ingredients that may share a similar composition, function, or purpose) might be used to characterize a food as ultra-processed?
Further, ingredients that function as flavorings are either natural flavors or artificial flavors; colorings are either certified (for instance, “FD&C Red No. 40”) or non-certified (for instance, “colored with beet juice”) (21 CFR 101.22). Should these various types of flavors and colors be considered separately when characterizing a food as ultra-processed? Please provide supporting data and explain your rationale in your response. - To what extent, if any, should the relative amount of an ingredient used in a food influence whether the food should be characterized as ultra-processed? Please provide supporting data and explain your rationale in your response.
- What, if any, other ingredients or ingredient-related criteria not discussed previously should or should not be used to characterize a food as ultra-processed? Please provide supporting data and explain your rationale in your response.
- FDA defines “manufacturing/processing,” in part, to mean making food from one or more ingredients, or synthesizing, preparing, treating, modifying, or manipulating food, including food crops or ingredients (21 CFR 117.3; see also 21 USC 321(gg) for the statutory definition of “processed food”). Certain FDA regulations, such as standards of identity, may prescribe methods of production or formulation (see, e.g., 21 CFR part 133). Processing of a food is often achieved by a combination of physical, biological, and chemical methods; however, while processing information is sometimes found on food labeling, manufacturers are not always required to disclose processing information on food labeling. The following questions focus on the processing of an ingredient or a mixture of ingredients into the finished food and whether certain processing methods may contribute to a food being considered ultra-processed.
- Processing a food through physical means may include cutting, extracting juice by an application of force, heating, freezing, extrusion, and other physical manipulations. What physical processes might be used to characterize a food as ultra-processed? Please provide supporting data and explain your rationale in your response.
- Processing a food through biological means may include non-alcoholic fermentations of the food by microorganisms (for example, bacteria and yeasts), enzymatic treatment, and other biological manipulations. What biological processes might be used to characterize a food as ultra-processed? Please provide supporting data and explain your rationale in your response.
- Processing a food through chemical means may include pH adjustment and other chemical manipulations. What chemical processes might be used to characterize a food as ultra-processed? Please provide supporting data and explain your rationale in your response.
- What, if any, other processing-related techniques should or should not be used to characterize a food as ultra-processed? Please provide supporting data and explain your rationale in your response.
- Is the term “ultra-processed” the best term to use, or is there other terminology that would better capture the concerns associated with these products? If there is another term to consider, please name and define that term and provide specific scenarios and citations (if available) to support its use.
- FDA and USDA are aware of ongoing research on nutrition and other attributes relating to the health outcomes associated with consumption of UPFs. As noted in the background, FDA is also initiating a joint effort with NIH to answer questions such as how and why UPFs can harm people's health.
- In considering nutritional attributes (such as information presented on the Nutrition Facts label), to what extent, if any, and how, should nutritional composition or the presence of certain nutrients be incorporated in a definition of UPFs? Please provide supporting data and explain your rationale in your response.
- What other attributes, such as energy density or palatability, might be used to characterize a food as ultra-processed? Please provide supporting data and explain your rationale in your response. If relevant to your answer, please also provide suggestions on how these attributes can be measured and/or potentially be incorporated into a definition of UPFs, if they are not readily apparent on the food labeling.
- FDA and USDA are exploring whether and how to incorporate various factors, such as the ones discussed in the questions above, into a uniform definition of UPFs. How might these factors be integrated in the classification of a food as ultra-processed in a way that can be systematically measured and applied to foods sold in the U.S.? And what considerations should be taken into account in incorporating such a classification in food and nutrition policies and programs?
III. References
The following references marked with an asterisk (*) are on display at the Dockets Management Staff and are available for viewing by interested persons between 9 a.m. and 4 p.m., Monday through Friday; they also are available electronically at https://www.regulations.gov. References without asterisks are not on public display at https://www.regulations.gov because they have copyright restriction. Some may be available at the website address, if listed. References without asterisks are available for viewing only at the Dockets Management Staff. Although FDA verified the website addresses in this document, please note that websites are subject to change over time.
- * Murphy, S.L., Kochanek, K.D., et al., “Mortality in the United States, 2023.” NCHS Data Brief, No. 521. Hyattsville, MD: National Center for Health Statistics. 2024. Accessed June 6, 2025. Available at https://stacks.cdc.gov/view/cdc/170564.
- Lane M.M., Davis, J.A., et al., “Ultraprocessed food and chronic noncommunicable diseases: a systematic review and meta-analysis of 43 observational studies.” Obesity Reviews. 2021;22(3):e13146. Accessed June 6, 2025. Available at https://doi.org/10.1111/obr.13146.
- Cordova R., Viallon, V., et al., “Consumption of ultra-processed foods and risk of multimorbidity of cancer and cardiometabolic diseases: a multinational cohort study.” Lancet Regional Health Europe. 2023;35:100. Accessed June 6, 2025. Available at https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(23)00190-4/fulltext.
- Lane M.M., Gamage, E., et al., “Ultra-processed food exposure and adverse health outcomes: umbrella review of epidemiological meta-analyses.” BMJ. 2024;384:e077310. Accessed June 6, 2025. Available at https://doi.org/10.1136/bmj-2023-077310.
- Hall, K.D., Ayuketah, A., et al., “Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake.” Cell Metabolism. 2019; 30:67-77. Accessed June 2, 2025. Available at: https://doi.org/10.1016/j.cmet.2019.05.008.
- Popkin, B., Miles, D., et al., “A policy approach to identifying food and beverage products that are ultra-processed and high in added salt, sugar and saturated fat in the United States: a cross-sectional analysis of packaged foods,” The Lancet Regional Health—Americas. 2024; 32: 100713. Accessed June 2, 2025. Available at https://doi.org/10.1016/j.lana.2024.100713.
- * Make America Healthy Again Commission, “The MAHA Report: Make Our Children Healthy Again,” The White House. 2025. Accessed June 2, 2025. Available at https://www.whitehouse.gov/maha/.
- Juul, F., Parekh, N., Martinez-Steele, E., et al., “Ultra-processed food consumption among US adults from 2001 to 2018,” The American Journal of Clinical Nutrition. 2022; 115: 211-221. Accessed June 2, 2025. Available at https://doi.org/10.1093/ajcn/nqab305.
- Wang, L., Martinez-Steele, E., et al., “Trends in Consumption of Ultraprocessed Foods Among US Youths Aged 2-19 Years, 1999-2018,” Journal of the American Medical Association. 2021; 326(6):519-530. Accessed June 2, 2025. Available at https://doi.org/10.1001/jama.2021.10238.
- * U.S. Food and Drug Administration and National Institutes for Health (NIH). “FDA and NIH Announce Innovative Joint Nutrition Regulatory Science Program.” Accessed June 2, 2025. Available at https://www.fda.gov/news-events/press-announcements/fda-and-nih-announce-innovative-joint-nutrition-regulatory-science-program.
- Gibney, M.J., “Ultra-Processed Foods: Definitions and Policy Issues.” Current developments in nutrition. 2019; 3:nzy077. Accessed June 2, 2025. Available at https://doi.org/10.1093/cdn/nzy077.
- Crino, M., Barakat T., et al., “Systematic Review and Comparison of Classification Frameworks Describing the Degree of Food Processing,” Nutrition and Food Technology. 2017; 3(1). Accessed June 2, 2025. Available at http://dx.doi.org/10.16966/2470-6086.138.
- de Araújo, T.P., de Moraes, M.M., et al., “Food Processing: Comparison of Different Food Classification Systems,” Nutrients. 2022; 14: 729. Accessed June 2, 2025. Available at https://doi.org/10.3390/nu14040729.
- Monteiro, C.A., “Nutrition and Health. The Issue Is Not Food, nor Nutrients, so Much as Processing.” Public Health Nutrition. 2009; 12: 729-731. Accessed June 2, 2025. Available at https://doi.org/10.1017/S1368980009005291.
- Monteiro, C.A., Cannon, G., et al., “Ultra-processed foods, diet quality, and health using the NOVA classification system,” Food and Agriculture Organization of the United Nations. 2019. Accessed June 5, 2025. Available at https://openknowledge.fao.org/bitstreams/5277b379-0acb-4d97-a6a3-602774104629/download.
- Monteiro, C.A., Cannon, G., et al., “Ultra-Processed Foods: What They Are and How to Identify Them.” Public Health Nutrition. 2019; 22: 936-941. Accessed June 2, 2025. Available at https://doi.org/10.1017/S1368980018003762.
- Monteiro C.A., Cannon G., et al., “The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing,” Public Health Nutrition. 2018; 21(1):5-17. Accessed June 2, 2025. Available at https://doi.org/10.1017/s1368980017000234.
- * U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th ed. 2020. Accessed June 2, 2025. Available at https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf.
FDA and USDA (we) are requesting data and information to help develop a uniform definition of ultra-processed foods (UPF or UPFs) for human food products in the U.S. food supply. A uniform UPF definition, developed as part of a joint effort by federal agencies, would allow for consistency in research and policy to pave the way for addressing health concerns associated with the consumption of UPFs.
Secretary Rollins Praises President Trump’s One Big Beautiful Bill
(Washington, D.C., July 4, 2025) – U.S. Secretary of Agriculture Brooke L. Rollins issued the following statement after President Donald J. Trump signed the One Big Beautiful Bill into law:
“The One Big Beautiful Bill marks the start of a new golden age for America and American agriculture. This historic piece of legislation makes permanent the largest tax cuts in history.
“It provides immediate tax relief to farmers, ranchers, and rural Americans by increasing the small business expensing threshold and permanently extending the Small Business Deduction. Through the President’s leadership, the bill Makes Agriculture Great Again, bolsters the farm safety net, makes crop insurance more affordable, and protects two million family farms from the death tax.
“It puts American Farmers First by preventing countries such as China and Brazil from flooding our markets with biofuel feedstocks that compete with American grown soy, milo, and corn. It extends the 45Z clean fuel tax credit to enhance our domestic energy security.
“While expanding programs to support the farmers who feed, fuel, and clothe America, this legislation also tackles the fraud and waste that has run rampant in the Supplemental Nutrition Assistance Program (SNAP). The bill holds states accountable for their error rates, strengthens work requirements, and prevents illegal aliens from receiving SNAP.
“President Trump’s One Big Beautiful Bill is a win for farmers, ranchers, rural communities, and American taxpayers. His leadership on this landmark piece of legislation is yet another example of an America First promise made and a promise kept,” said Secretary Rollins.
###
ICYMI: Secretary Rollins Op-Ed in Newsweek “Farmers Win With the One Big Beautiful Bill”
Washington, D.C., June 30, 2025 – U.S. Secretary of Agriculture Brooke L. Rollins published an opinion piece in Newsweek on how the One Big Beautiful Bill (OBBB) will empower farmers and ranchers.
“Over the next decade, the OBBB increases the farmer safety net, crop insurance, and trade programs. These changes to farm support will allow USDA to return to our core mission of putting Farmers First and restoring rural prosperity across the country. At USDA, we are working every day on behalf of our farmers and ranchers to ensure President Trump's America First agenda unleashes prosperity for generations to come,” said Secretary Rollins.
Read the full piece below.
Our nation's farmers and ranchers are ready to put America First. Over 97 percent of our agricultural counties voted for President Donald Trump last November, and he will never forget their commitment to his agenda.
Now is the time to put that agenda into effect. The One Big Beautiful Bill (OBBB) unleashes economic growth and restores fiscal sanity.
With the largest tax cut for middle- and working-class Americans in history, an increased child tax credit, new Trump savings account for newborns, historic tax relief for seniors, and no tax on tips and overtime, every American family wins with the OBBB.
And this bill will serve no one more than our nation's farm families. The OBBB cuts taxes for farmers by over $10 billion and prevents the death tax from hitting 2 million family-owned farms that would otherwise see their exemptions cut in half.
Along with bolstering the farm safety net for producers to protect against significant economic losses, which has not been updated since 2014, the OBBB also makes risk management tools more affordable at a time of significant risk because of the previous administration's neglect of our rural communities.
The bill also helps the 98 percent of farming operations that are taxed according to their owners' individual tax rate by protecting the small business deduction. Because the agricultural industry leverages one-fifth of all small business deductions across the American economy, the OBBB's doubling of Section 179 deductions to $2.5 million will provide major relief for farming operations across the country. The extension of the Section 199A Qualified Business Income Deduction will also save farmers thousands of dollars every year.
The OBBB also realigns the Supplemental Nutrition Assistance Program (SNAP) to President Trump's mission of cutting waste, fraud, and abuse across the federal bureaucracy. On any given day, USDA spends nearly $265 million on SNAP benefits alone, not including the 15 other nutrition programs at the department. In 2023, over $11 billion in erroneous SNAP payments was issued. The bill is centered on increased accountability, on both the individual recipient and the state administering the program.
To address this waste of taxpayer dollars, the OBBB implements a state cost-share program proportionate to state payment error rates, which will incentivize states to distribute SNAP funds only to those who qualify. The bill also expands work requirements for able-bodied adults without dependents. Welfare should be a helping hand, not a pathway to lifelong dependency. With over 4 million jobs available across the country, SNAP work requirements will restore dignity through work. Under President Trump's leadership, this careless use of taxpayer money is ending so USDA can refocus its resources on revitalizing rural communities and uplifting the farm families that provide the food, fuel, and fiber for our great nation.
And the OBBB finally ends taxpayer subsidization of mass migration by ensuring only U.S. citizens or green card holders are eligible for SNAP. Our food assistance programs are funded by Americans for Americans. This bill puts the American people first by providing assistance to those in need while doing right by hardworking taxpayers.
Over the next decade, the OBBB increases the farmer safety net, crop insurance, and trade programs. These changes to farm support will allow USDA to return to our core mission of putting Farmers First and restoring rural prosperity across the country. At USDA, we are working every day on behalf of our farmers and ranchers to ensure President Trump's America First agenda unleashes prosperity for generations to come.
###
Letter to WIC State Health Commissioners
June 17, 2025
Dear State Health Commissioner,
Under the leadership of President Trump and Secretary Rollins, we have an opportunity to leverage the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) to Make America Healthy Again (MAHA).
The MAHA movement is tackling America’s chronic disease crisis, including diet-related diseases like childhood obesity and diabetes. With a proven track record of improving children’s health, WIC is an example of a government program that is focused exclusively on the nutritional health of its participants. It supports health-conscious food purchases by providing minimally processed, healthy foods needed to support healthy development during critical life stages—pregnancy, postpartum, lactating, infancy, and childhood.
I am writing today to request your commitment to review the foods on your WIC food lists and eliminate or reduce foods that contain artificial food colors, particularly petroleum-based synthetic dyes.
Recently, Secretary Rollins applauded the dairy industry’s voluntary commitment to remove artificial food colors from the National School Lunch Program. Participation in WIC is often a precursor for children participating in school meals programs. We have a joint responsibility to extend this commitment and mitigate early exposure to these food colors during infancy and early childhood—a period of rapid growth and development.
To further support consumers, the U.S. Department of Health and Human Services, Food and Drug Administration, under Secretary Kennedy’s leadership, approved three food colors from natural sources and is working with industry to phase out petroleum-based synthetic food dyes in the nation’s food supply. I encourage you to take action ahead of the planned phase out and support us in implementing the Administration’s future actions within WIC.
I look forward to working with you to Make America Healthy Again.
Sincerely,
James C. Miller
Administrator
Food and Nutrition Service
United States Department of Agriculture
Under the leadership of President Trump and Secretary Rollins, we have an opportunity to leverage the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) to Make America Healthy Again.
Information Collection - WIC Tribal Organizations and U.S. Territories Study
Summary
In accordance with the Paperwork Reduction Act of 1995, this notice invites the general public and other public agencies to comment on this proposed information collection for the WIC Tribal Organizations and U.S. Territories Study. This is a new information collection request. The U.S. Department of Agriculture's Food and Nutrition Service (FNS) administers the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) program to improve the health of nutritionally at-risk women and children. WIC is one of the nation's most successful public health nutrition programs. This study involves all 32 Indian Tribal Organizations (ITOs, “Tribal Organizations”) and 5 U.S. territories that operate WIC state agencies, five tribally operated local WIC agencies, and approximately 15 geographic state agencies that share a border with a Tribal Organization. This study aims to inform FNS about variations in operations among Tribal Organizations, U.S. territories, and geographic states administering WIC as local and state agencies. The results of the study may inform efforts to improve WIC program operations and participant services.
Request for Comments
Written comments must be received on or before Aug. 12, 2025.
Comments may be sent to Dr. Karen Castellanos-Brown, Food and Nutrition Service, U.S. Department of Agriculture, 1320 Braddock Place, Alexandria, VA 22314, or submitted via email at Karen.Castellanos-Brown@usda.gov. Comments will also be accepted through the Federal eRulemaking Portal. Go to http://www.regulations.gov and follow the online instructions for submitting comments electronically.
All responses to this notice will be summarized and included in the request for Office of Management and Budget approval. All comments will be a matter of public record.
Abstract
The Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) improves the health of nutritionally at-risk women and children and is recognized as one of the nation's most successful public health nutrition programs. Eighty-eight state agencies, including 5 U.S. territories (Puerto Rico, Guam, the U.S. Virgin Islands, American Samoa, and the Commonwealth of the Northern Mariana Islands) and 32 Indian Tribal Organizations (ITOs, “Tribal Organizations”), currently administer WIC. While WIC program requirements are the same across all WIC state agencies, there is flexibility in adapting the program to the local context. This flexibility can create variation in the experiences of WIC program staff and participants. For instance, WIC agencies from geographic states traditionally contract with local agencies to provide direct services to participants. However, nearly all WIC agencies in Tribal Organizations and U.S. territories conduct state agency-level functions and provide direct WIC services.
FNS is conducting the WIC Tribal Organizations and U.S. Territories Study to learn more about how WIC agencies in Tribal Organizations and U.S. territories administer and operate the program. The results will inform FNS about differences in WIC program operations and experiences among Tribal Organizations, U.S. territories, and geographic state agencies and can inform possible program improvements. Study objectives include:
- Comprehensively describe WIC program administration and operations among ITOs and territories operating as state and local agencies.
- Understand the facilitators and barriers to WIC program administration and operations among ITOs and territories operating state and local agencies.
- Examine and describe the differences in WIC state agency-level policy, service delivery, and operations between ITOs and U.S. territories and geographic state agencies.
- Obtain information to inform determination of WIC coverage rates among ITOs and territories.
The study takes a qualitative case study approach and will gather information from the following sources: (a) extant data; (b) interviews with WIC directors (all 32 state agency-level Tribal Organizations, all five Tribally operated local WIC agencies, and 15 geographic state agencies); and (c) site visits to 20 selected Tribal Organizations and U.S. territories. Site visits will include interviews with WIC clinic staff and observations of the clinic environment, participant appointments, and WIC-authorized retailers.1
1 Observations of the clinic environment and WIC-authorized retailers are passive observations in public space and will not involve burden on participants.
This notice invites the general public and other public agencies to comment on this proposed information collection for the WIC Tribal Organizations and U.S. Territories Study. This is a new information collection request.
Comment Request: WIC & FMNP Outreach, Innovation, and Modernization Evaluation
Summary
The American Rescue Plan Act of 2021 (ARPA), which was signed into law in March 2021, provided USDA with $390 million and waiver authority for outreach, innovation, and program modernization in WIC and the WIC Farmers' Market Nutrition Program (FMNP). FNS is interested in understanding the implementation and outcomes related to these modernization efforts. This information collection request (ICR) is for the 2023-2028 WIC & FMNP Outreach, Innovation, and Modernization Evaluation (WIC modernization evaluation).
The Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) provides supplemental food, nutrition education, and referrals to health and social services to pregnant and postpartum women, infants, and children up to age 5 who are living in households with low incomes and are at nutritional risk. The Farmers Market Nutrition Program (FMNP) provides eligible WIC participants with FMNP benefits, in addition to their regular WIC benefits, which can be used to buy eligible foods from authorized outlets, including farmers, farmers' markets, or roadside stands.
The WIC modernization evaluation has three components: an implementation study, a waiver study, and an impact study.
Request for Comments
Comments regarding this information collection received by July 11, 2025 will be considered. Written comments and recommendations for the proposed information collection should be submitted within 30 days of the publication of this notice on the following website www.reginfo.gov/public/do/PRAMain. Find this particular information collection by selecting “Currently under 30-day Review—Open for Public Comments” or by using the search function.
Need and Use of the Information
The implementation study will provide a comprehensive understanding of project implementation while accommodating variations in the timing of projects within different program areas, implementation within and between state agencies, and innovative approaches. The implementation study will collect data from WIC state agencies, local agencies, clinics, WIC & FMNP vendors and outlets, and WIC participants. These data will provide current and ongoing information about modernization efforts in all 88 WIC state agencies.
The waiver study will provide an understanding of waiver issuance and use. The waiver study will rely on many of the same data sources as the implementation study, especially with WIC state agencies. The study will also collect information on whether and how unique waivers were implemented by WIC state agencies to conduct the modernization projects.
The impact study will measure the impact of the WIC and FMNP modernization projects on participants through key short-term and intermediate-term outcome measures. It will address whether the modernization projects improved key outcome measures and how changes in these outcomes were related to the number and type of modernization projects. Most outcomes will be measured with administrative data. The impact study will also use surveys to learn about the experiences and satisfaction of WIC program staff, WIC & FMNP vendor/outlet staff, and participants with the changes to the WIC program as a result of the modernization activities. In addition, the impact study will rely on information from the implementation and waiver studies about where and when projects and waivers were implemented.
The American Rescue Plan Act of 2021 (ARPA), which was signed into law in March 2021, provided USDA with $390 million and waiver authority for outreach, innovation, and program modernization in WIC and the WIC Farmers' Market Nutrition Program. FNS is interested in understanding the implementation and outcomes related to these modernization efforts.
Secretary Rollins Signs State Waivers to Make America Healthy Again by Removing Unhealthy Foods from SNAP in Arkansas, Idaho, and Utah in Addition to Indiana, Iowa, and Nebraska
Washington, D.C., June 10, 2025 – U.S. Secretary of Agriculture Brooke L. Rollins, joined by Secretary of Health and Human Services Robert F. Kennedy Jr., signed three new food choice waivers to Make America Healthy Again. The signed waivers will amend the statutory definition of food for purchase for Supplemental Nutrition Assistance Programs (SNAP) in Arkansas, Idaho, and Utah, each commencing in 2026.
“The Trump Administration is unified in improving the health of our nation. America’s governors have proudly answered the call to innovate by improving nutrition programs, ensuring better choices while respecting the generosity of the American taxpayer. Each waiver submitted by the states and signed is yet another step closer to fulfilling President Trump’s promise to Make America Healthy Again,” said Secretary Rollins.


“Thank you to the governors of Indiana, Arkansas, Idaho, Utah, Iowa, and Nebraska for their bold leadership and unwavering commitment to Make America Healthy Again,” said Secretary Kennedy. “I call on every governor in the nation to submit a SNAP waiver to eliminate sugary drinks—taxpayer dollars should never bankroll products that fuel the chronic disease epidemic.”
Secretary Rollins and Secretary Kennedy were joined at the event by Governor of Arkansas Sarah Huckabee Sanders and Governor of Indiana Mike Braun.
“This approval sends a clear message: President Trump and his administration are tackling America’s chronic disease epidemic and Arkansas stands with him in that fight,” said Governor Sanders. “I am incredibly grateful for Secretary Rollins’ quick approval of our waiver. Arkansas leads the nation in getting unhealthy, ultra-processed foods off food stamps and helping our most vulnerable citizens lead healthier lives.”
“Indiana is proud to be a leader in the Make America Healthy Again initiative, and I'm proud to join Secretary Rollins, Secretary Kennedy, Congressman Baird, and my fellow Governor Sarah Huckabee Sanders today to discuss returning SNAP to its proper purpose of nutrition, and how my Make Indiana Healthy Again agenda supports Indiana agriculture and empowers Hoosiers to live longer, healthier lives,” said Governor Braun.
Prior to these waivers, SNAP recipients could buy anything except alcohol, tobacco, hot and prepared foods, and personal care products. This historic action expands the list of products excluded from SNAP purchases in Arkansas, Idaho, and Utah. Arkansas’ waiver excludes soda, low and no-calorie soda, fruit and vegetable drinks with less than 50% natural juice, other unhealthy drinks, and candy, and it will take effect July 1, 2026. The waiver for Idaho excludes soda and candy, and it will take effect Jan. 1, 2026. The waiver for Utah excludes soft drinks, and it will take effect Jan. 1, 2026. Secretary Rollins has previously signed waivers for Nebraska, Iowa, and Indiana.
"Idaho proudly welcomes the MAHA movement because it is all about looking for new ways to improve nutrition, increase exercise, and take better care of ourselves and one another, especially our children. We are excited to partner with the Trump administration in bringing common sense to the government's food assistance program with the approval of our SNAP waiver," said Governor Brad Little.
At the direction of President Trump, Secretary Rollins is ensuring programs work harder to encourage healthy eating and improved lifestyle habits while protecting taxpayer dollars. On Secretary Rollins’ first full day in office, she sent a letter to the nation’s governors (PDF, 88.8 KB), outlining her vision for the Department and inviting them to participate in a new “Laboratories of Innovation” initiative to create bold solutions to long-ignored challenges. Secretary Rollins and Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. wrote an opinion piece in USA Today outlining their plan to Make America Healthy Again, including through SNAP waivers like the ones signed today.
###
Proposed Information Collection: Generic Clearance To Conduct Formative Research or Development of Nutrition Education and Promotion Materials and Related Tools and Grants for FNS Population Groups
Summary
In accordance with the Paperwork Reduction Act of 1995, this notice invites the general public and other interested parties to comment on a proposed information collection. This collection is an extension of a currently approved collection. This information collection will conduct research in support of FNS' goal of delivering science-based nutrition education to targeted audiences. This information collection will also conduct research that will assist FNS in identifying effective design and implementation approaches to use to develop and assess grants. From development through testing of materials and tools with the target audience, FNS plans to conduct data collections that involve formative research including focus groups, interviews (dyad, triad, telephone, etc.), surveys and Web-based collection tools.
Request for Comments
Written comments must be received on or before July 28, 2025.
Comments may be sent to the Planning and Regulatory Affairs Office, Food and Nutrition Service, U.S. Department of Agriculture, 1320 Braddock Place, 5th floor, Alexandria, VA 22314. Comments may also be sent via fns-prao@usda.gov.
Comments will also be accepted through the Federal eRulemaking Portal. Go to https://www.regulations.gov, and follow the online instructions for submitting comments electronically. All responses to this notice will be summarized and included in the request for Office of Management and Budget (OMB) approval. All comments will be a matter of public record.
Abstract
This information collection is based on section 19 of the Child Nutrition Act of 1966 (42 USC 1787), section 5 of the Richard B. Russell National School Lunch Act (42 USC 1754) and section 11(f) of the Food and Nutrition Act of 2008 (7 USC 2020). This request for approval of information collection is necessary to obtain input into the development of nutrition education interventions for population groups served by the U.S. Department of Agriculture, Food and Nutrition Service (USDA-FNS). FNS also uses this collection to obtain input that can be used to develop and assess grants. Interventions need to be designed so that they can be delivered through different types of media and in a variety of formats for diverse audiences.
FNS develops a variety of resources to support nutrition education and promotion activities. These resources are designed to convey science-based, behavior-focused nutrition messages about healthy eating and physical activity to children and adults eligible to participate in FNS nutrition assistance programs and to motivate them to consume more healthful foods as defined by the Dietary Guidelines for Americans (DGA). This includes education materials, messages, promotion tools and interventions for the diverse population served by the federal nutrition programs as well as WIC, Team Nutrition, Food Distribution and other programs.
Obtaining formative input and feedback is fundamental to FNS' success in delivering science-based nutrition messages and reaching diverse segments of the population in ways that are meaningful and relevant. This includes conferring with the target audience, individuals who serve the target audience, and key stakeholders on the communication strategies and interventions that will be developed and on the delivery approaches that will be used to reach consumers. The formative research and testing activities described will help in the development of effective education and promotion tools and communication strategies. Collection of this information will increase FNS' ability to formulate nutrition education interventions that resonate with the intended target population, particularly low-income families.
FNS also uses formative input and feedback to determine how best to develop and assess grants so that grant recipients can successfully meet their goals under these grants. To do this, FNS confers with grant recipients to obtain input regarding their experiences, expectations, challenges, and lessons learned while implementing the grant.
Formative research methods and information collection will include focus groups, interviews (dyad, triad, telephone, etc.), surveys and web-based data collection. The data obtained will provide input regarding the potential use of materials and products during both the developmental and testing stages, in addition to the development of grants. Key informant interviews will be conducted in order to determine future nutrition education and grant needs, tools and dissemination strategies. This task involves collecting a diverse array of information from a variety of groups including: people familiar with the target audiences; individuals delivering nutrition education intervention materials and projects; program providers at state and local levels; program participants; grant recipients, and other relevant informants associated with FNS programs.
Findings from all data collection will be included in summary reports submitted to USDA-FNS. The reports will describe the data collection methods, findings, conclusions, implications, and recommendations for the development and effective dissemination of nutrition education materials and related tools for FNS population groups. There will be no specific quantitative analysis of data. No attempt will be made to generalize the findings to be nationally representative or statistically valid. There are no recordkeeping or third party disclosure burden requirements.
In accordance with the Paperwork Reduction Act of 1995, this notice invites the general public and other interested parties to comment on a proposed information collection. This collection is an extension of a currently approved collection to conduct research in support of FNS' goal of delivering science-based nutrition education to targeted audiences.
Secretary Rollins Approves State Waivers to Make America Healthy Again by Removing Unhealthy Foods from SNAP in Indiana and Iowa
Washington, D.C., May 23, 2025 – U.S. Secretary of Agriculture Brooke L. Rollins signed waivers to amend the statutory definition of food for purchase for Supplemental Nutrition Assistance Programs (SNAP) in Indiana and Iowa, each commencing in 2026. On Monday, Secretary Rollins signed the first-ever waiver of this kind in Nebraska.
“President Trump has given our nation a once in a generation opportunity to change the health trajectory for our entire country. On my first day as Secretary, I sent a call to states to innovate, and Governors Jim Pillen, Kim Reynolds, Mike Braun, Sarah Huckabee Sanders, Laura Kelly, Patrick Morrisey, Jared Polis, Brad Little, Spencer Cox, and Greg Abbott have stepped up and taken action. I look forward to signing even more waivers in the days ahead as we continue to restore the health of America,” said Secretary Rollins.
“Indiana is proud to be a leader in the Make America Healthy Again initiative, and today Secretary Rollins signed our waiver to return SNAP in Indiana to its intended purpose: nutrition. President Trump and Secretary Rollins are putting our farmers first and supporting American agriculture, and I was proud to join them today,” said Governor Braun.
“Soaring obesity rates have brought our nation and state to a crossroads,” said Governor Reynolds. “To promote healthy eating and protect future generations from disease—and to ensure SNAP fulfills its core function—we need a change. Thank you to Secretary Rollins and her team for helping make that change happen.”
Prior to these waivers, SNAP recipients could buy anything except alcohol, tobacco, hot foods, and personal care products. This historic action expands the list of products excluded from SNAP purchases in Indiana and Iowa. Indiana’s waiver excludes soft drinks and candy, and it will take effect Jan. 1, 2026. The waiver for Iowa excludes any food item eligible for sales tax including sweetened beverages, snacks, and candy, and this waiver will take effect Jan. 1, 2026.
As part of the Make America Healthy Again agenda, this historic action seeks to reverse alarming disease trends across the country. Prediabetes now affects one in three children ages 12 to 19; 40% of school-aged children and adolescents have at least one chronic condition; and 15% of high school students drink one or more sodas daily.
At the direction of President Trump, USDA is ensuring programs work harder to encourage healthy eating and improved lifestyle habits. On Secretary Rollins’ first full day in office, she sent a letter to the nation’s governors (PDF, 88.8 KB), outlining her vision for the Department and inviting them to participate in a new “Laboratories of Innovation” initiative to create bold solutions to long-ignored challenges. Secretary Rollins and Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. wrote an opinion piece in USA Today outlining their plan to Make America Healthy Again, including through SNAP waivers like the one signed today.
###
WIC Infant and Toddler Feeding Practices Study-2: Ninth Year Report
The WIC Infant and Toddler Feeding Practices Study-2 (WIC ITFPS-2), also known as the "Feeding My Baby Study," is the only national study to analyze the long-term impact of WIC by gathering information on caregivers and children over the first nine years of the child's life after enrollment in WIC, regardless of their continued participation in the program.
WIC Participation Is Associated With Declining Rates of Hunger
- Over the course of the nine-year study, household hunger among study participants declined dramatically from baseline (during pregnancy or shortly after birth) to age 6 (from 48% to 22%) and then rose slightly to 26% at age 9 (Figure 1).
- Although the study children were no longer eligible to receive WIC at age 9, nearly 20% of their caregivers received WIC for themselves or another child or both.
- Households with at least one WIC participant when the study child was 9 years old were less likely to report experiencing household hunger, as well as child hunger, compared to those not participating in WIC (Figure 2).


WIC Caregivers Choose Healthier Foods for Their Families
- Four out of every five study caregivers reported that they learned something from WIC that helps them make decisions about what foods to offer their 9-year-old child.
- Around 40% of study caregivers said they learned how to choose healthier foods for their 9-year-old child and 38% of caregivers indicated their families eat more fruits and vegetables because of something they learned from WIC (Figure 3).
- Caregivers who reported at year nine that they learned something from WIC that helped them make decisions about the foods they offer to the study child were more likely to say they often or very often had fruits, dark green vegetables, and reduced fat milks available in their home compared to caregivers who said they didn’t learn something from WIC.

WIC Participation Beyond Age 3 is Linked to Higher Diet Quality1 in Young Children
- Looking at duration of WIC participation up to age 9, diet quality scores were higher for children who participated in WIC beyond age 3 compared to children who stopped participating before age 3 (Figure 4).
- This study found that 9-year-old children who previously participated in WIC had similar diet quality scores as the national average at ages 2, 3, 4, and 6 and significantly higher scores at ages 5 and 9, regardless of the duration of WIC participation.
- Consumption of nutrients of concern like saturated fat, added sugars, and sodium among study children was also similar to the general U.S. population at age 9.

Why FNS Did This Study
WIC is one of USDA, FNS's nutrition assistance programs. WIC safeguards the health of pregnant and postpartum women, infants, and young children from households with low incomes who are at nutritional risk. The WIC Infant and Toddler Feeding Practices Study-2 (WIC ITFPS-2), also known as the "Feeding My Baby Study," is the only national study to capture information about caregivers and their children over the first nine years of the child's life after enrollment in WIC, regardless of their continued participation in the program.
Little is known about the long-term impact of WIC participation on the health and diets of children who participate early in life. In 2024, we published the WIC ITFPS-2 Sixth Year Report showing that WIC participation is positively linked to diet quality among children. We extended WIC ITFPS-2 until the study children turned 9 years old. Continuing the study helped us better understand the longer-term diet, health, and food security status of children who participated in WIC as infants and young children.
How FNS Did This Study
Starting in July 2013, we enrolled 3,775 caregiver-child pairs in the study who were certified to participate in WIC during the caregiver’s pregnancy or shortly after the child’s birth. Caregivers and their children were enrolled in the study if the caregiver was at least 16 years old, spoke English or Spanish, and were certified at a WIC agency that enrolled at least 30 new pregnant women or infants per month.
Throughout the study, caregivers were interviewed every 2 to 6 months through the child’s fifth birthday, with follow-up interviews at ages 6 and 9 years. While many caregivers stayed in the study up to age 9, the analysis for the WIC ITFPS-2 Ninth Year report was made up of the 683 caregivers who completed every interview in the study – that is “the longitudinal sample”.
During the year 9 interview, caregivers completed a survey over the phone and a 24-hour dietary recall. To analyze the information provided by caregivers, we used statistical weights to inflate the sample to represent a national population of study-eligible caregivers and their children.
The interview questionnaires and recruitment materials for this study are available at RegInfo.gov. Publicly available data from pregnancy through age 5 can be found on Ag Data Commons.
Suggested Citation
Borger, C., Zimmerman, T. P., DeMatteis, J., Gollapudi, B., Thorn, B., Whaley, S.E., & Ritchie, L. (2025). WIC Infant and Toddler Feeding Practices Study-2: Year 9 report. Westat. U.S. Department of Agriculture, Food and Nutrition Service. This report was conducted by Westat under Contract No. GS-00F-009DA.
1 Overall diet quality was measured using the Healthy Eating Index Score (HEI). On a scale of 0 to 100, an HEI score of 100 means that the diet meets all the Dietary Guidelines for Americans recommendations.
This report, in the WIC Infant and Toddler Feeding Practices Study 2 (WIC ITFPS-2)/ “Feeding My Baby” Study analyzes the long-term impact of the USDA’s Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) by gathering information on caregivers and children over the first nine years of the child's life after enrollment in WIC, regardless of their continued participation in the program.
First 100 Days at FNS
President Trump made a commitment to the American people to cut wasteful spending, Make America Healthy Again, and to combat fraud, waste, and abuse—restoring common sense to government. Under the leadership of Secretary Rollins, USDA’s FNS has taken swift and decisive action to be representative of the change the American people voted for.